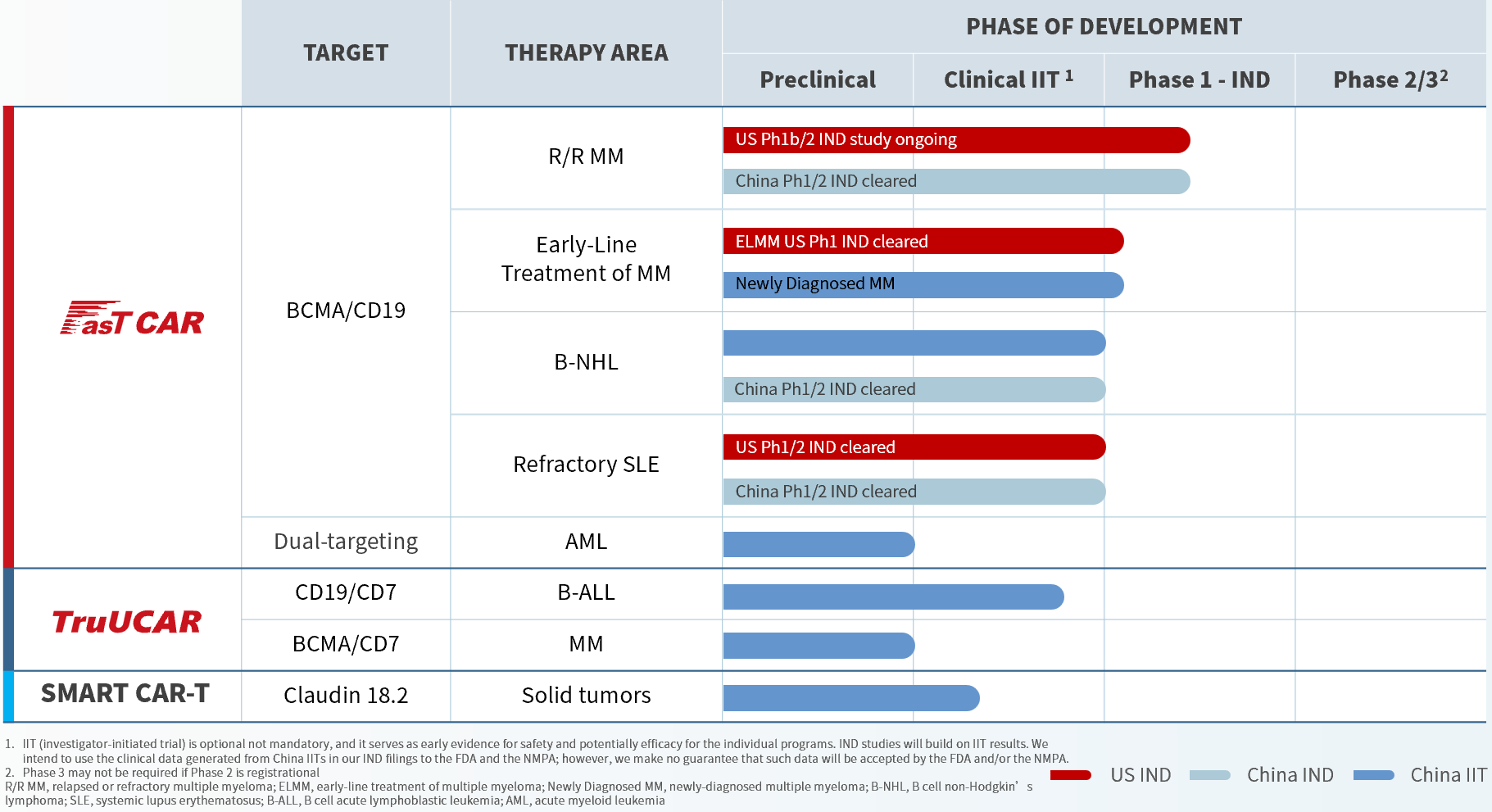
We are developing a rich pipeline of differentiated autologous and allogeneic product candidates. We seek to develop our product candidates by leveraging our relationships with clinicians and key opinion leaders in China, the United States and Europe. We partner with key hospitals in China to streamline the safety and efficacy testing of our innovative pipeline product candidates in investigator-initiated trials that are conducted in accordance with international standards to support future global regulatory filings and clinical development. We have generated all our product candidates internally. Our most advanced product candidates are presented in the pipeline diagram below:
Early access to AstraZeneca investigational medicinal products
We recognise that there are circumstances where patients with serious or life-threatening diseases have exhausted all available therapeutic options and may not be eligible to enroll in one of our clinical trials. In such circumstances individual patients may be eligible for early access to an AstraZeneca investigational medicinal product.
 Early Access Information
Early Access Information